Please note: This is the most overtly fraudulent study that I have ever seen in my 30+ years of reading research.
UPDATE DECEMBER 2024: JAMA/AMA has still failed to retract this horrid publication: https://jamanetwork.com/journals/jama/fullarticle/2776738
1. Imagine a study among acutely infected patients wherein the researchers delayed treatment for 18 days and then claimed that the antibiotic treatment was a failure—obviously the failure was in the design of the research and in the unethical and immoral delay in treatment.
2. Imagine a study among patients with life-threatening seizures (status epilepticus) wherein the researchers gave the correct treatment in the wrong way that resulted in a 15-day delay in effective treatment—obviously such research would be considered completely incompetent, immoral, and unethical.
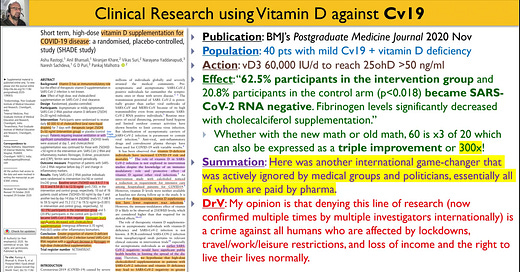
Publication: JAMA 2021 Feb (Murai et al)
Publisher: American Medical Association
Population: n=240 patients with Cv19
Actions and Failures:
PHARMACEUTICAL FAILURES: you should know by now that this was a study designed to fail as I have explained in video.
bolus 200,000 IU Vitamin D3 delivered orally in peanut oil,
while the D3 bolus needs 7 days to peak 25ohD levels,
amid a background of supraphysiologic D3 levels which antagonizes 25ohD, and
induces D3-clearance pathways (eg 24 hydroxylation)
ADMINISTRATIVE FAILURE: after an inexcusable delay of >11 days from the onset of illness for an illness that has a typical duration of 14 days
Effect: no benefit except to drug sales; bogus research contributes to the illness/death/lockdown/mandates of millions of people
Summation: This is the most critically important fraudulent trial that I have ever read; anyone who cites this article as evidence of anything other than fraud is complicit in fraud
DrV: AMA published bogus garbage to make nutrition appear ineffective, to support the drug/V paradigm, sacrificing the public health of hundreds of millions of people; call for retraction posted (20 February 2021) by DrV
Confirmation of hypocrisy #1: Imagine a study of emergency status epilepticus with intervention delay of 11 days and use of a slow-release form of antiseizure drugs which requires 7 days to become active; such a study = COMPLETELY UNETHICAL
Confirmation of hypocrisy #2: Imagine a study of acute bacterial infection with 18-day delay in ABX; such a study would never be approved by any competent IRB (institutional review board) nor published
**See full video at the link below**
VITAMIN D (Goldmine5) in Antimicrobial Antiviral Defense against Clinically Important Viral Infections
See video embedded above for “VITAMIN D (Goldmine5) in Antimicrobial and Antiviral Defense against Clinically Important Viral Infections”













Share this post